
Video Insights
Advertisement
Dr. Perez breaks down her study that investigated the use of ramucirumab and somatostatin in patients with advanced NETs.
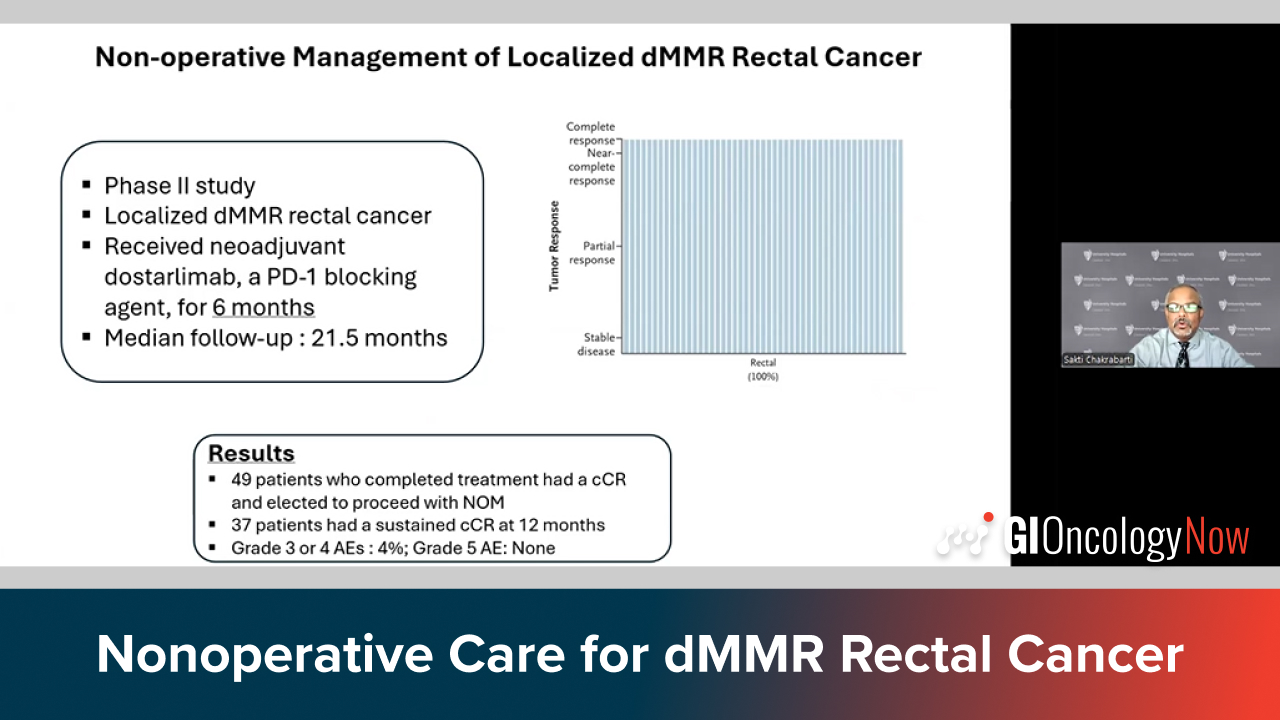
Dr. Chakrabarti highlights 100% response with PD-1 blockade in dMMR rectal cancer and new chemoimmunotherapy for anal cancer.
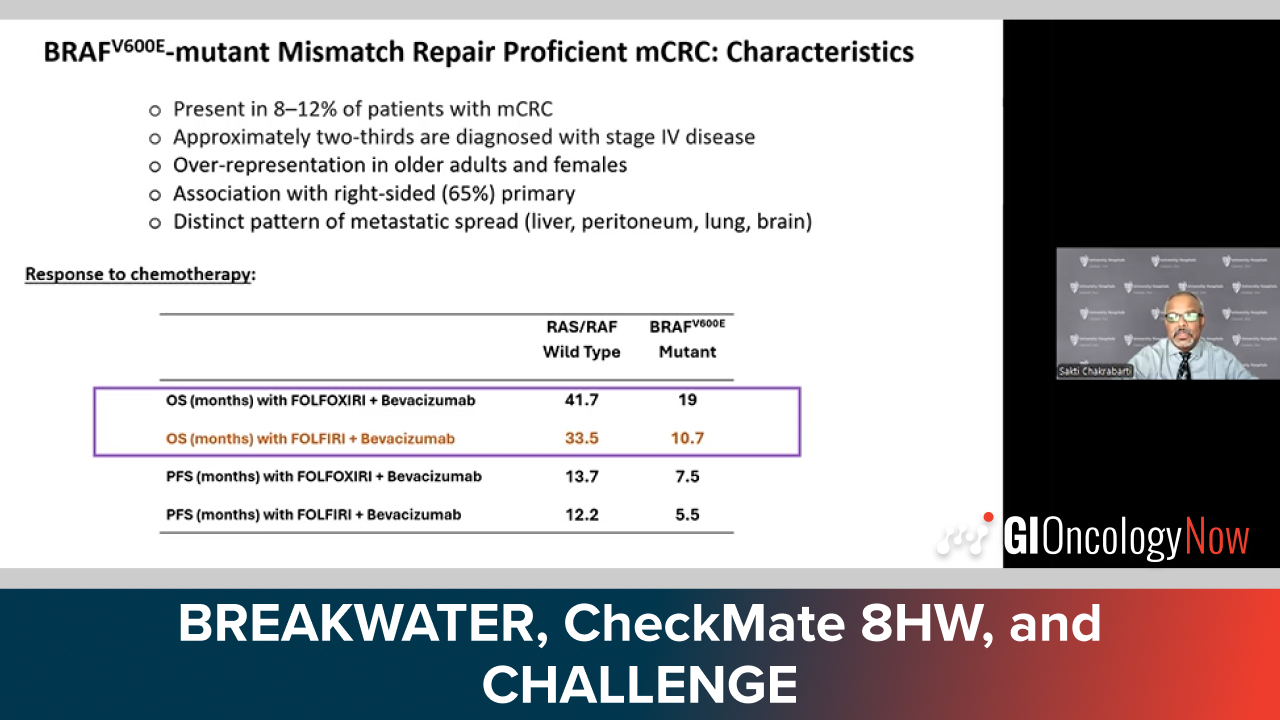
Dr. Chakrabarti shares BREAKWATER, CheckMate 8HW, and CHALLENGE results improving metastatic colorectal cancer outcomes.
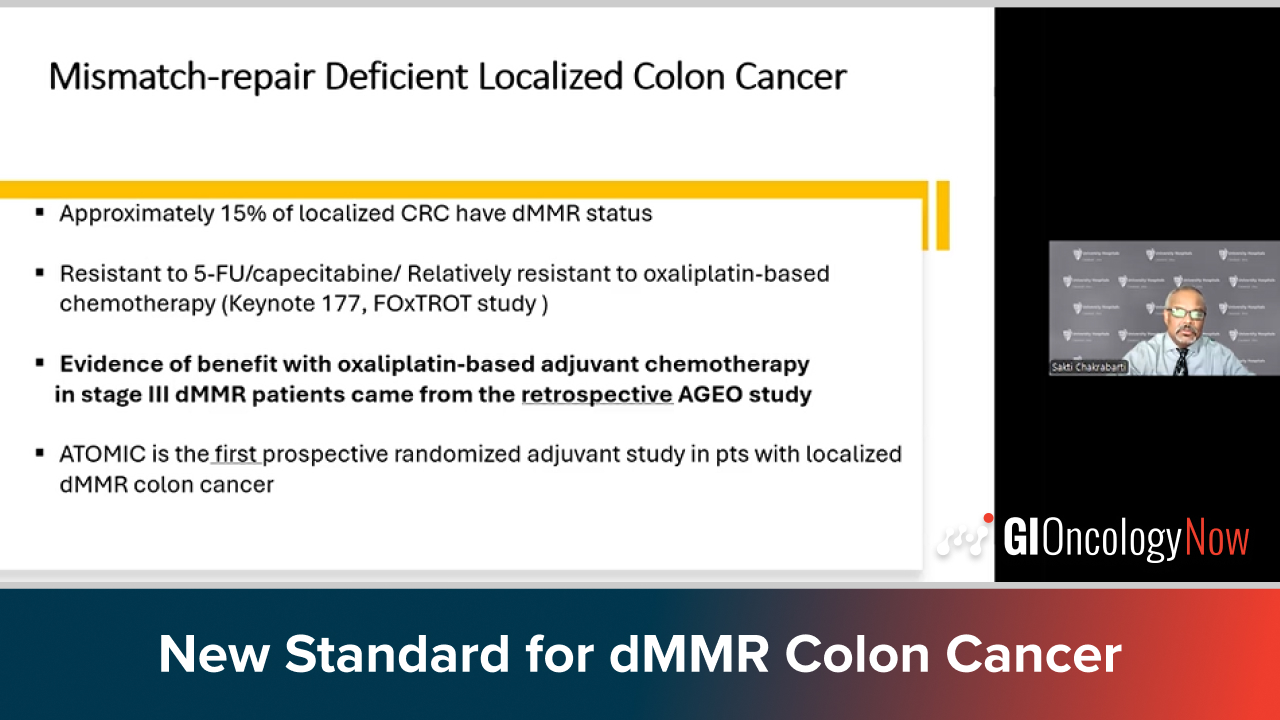
Dr. Chakrabarti highlights ATOMIC as the new standard for stage III dMMR colon cancer and compares it to the NICHE approach.
Dr. Lieu lists and describes DESTINY-Gastric04, CHALLENGE, MATTERHORN, BREAKWATER, and ATOMIC.
Dr. Goff shares how she chooses between atezo/bev and doublet IO regimens like durva/trem or ipi/nivo for patients with aHCC.
Dr. Kopetz explains how BREAKWATER aligns with Project FrontRunner to support accelerated approval in BRAF V600E-mutated CRC.
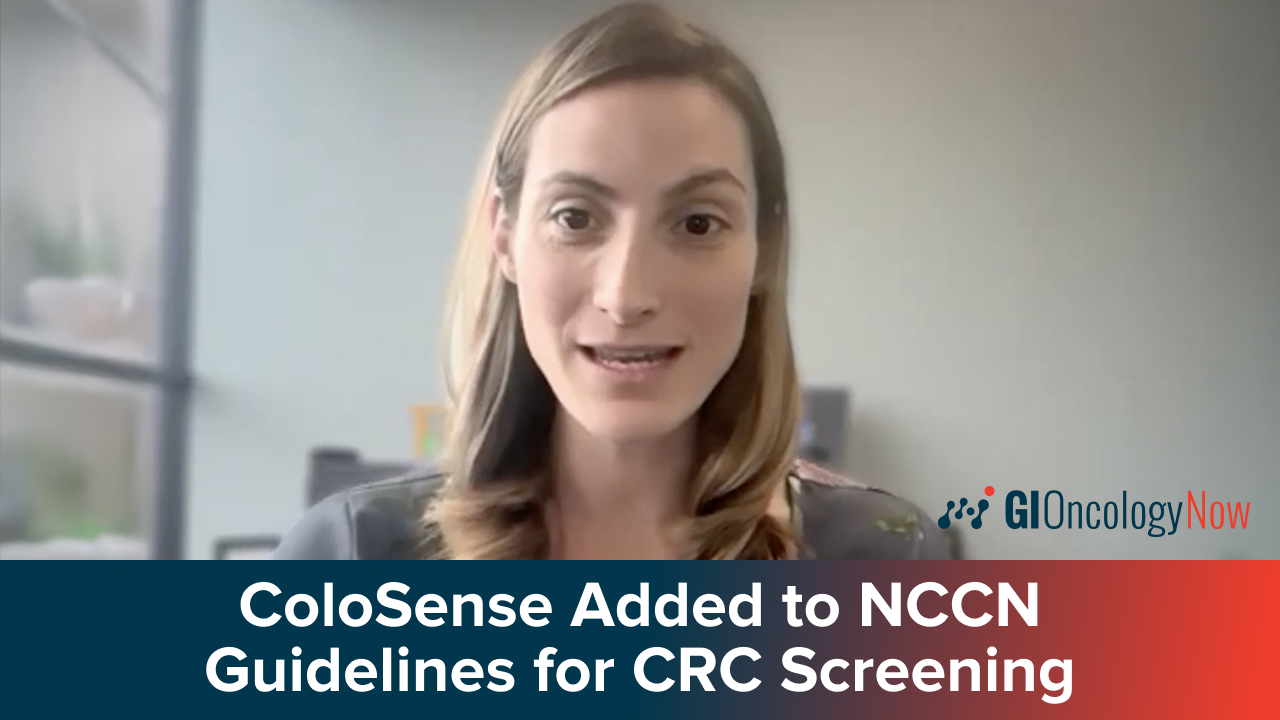
Dr. Barnell discusses ColoSense's NCCN Guidelines inclusion for CRC and how it impacts the cancer screening landscape.
Dr. He shares her insights on the evolving role of single-agent vs combination IO in the neoadjuvant setting for aHCC.
Dr. Janjigian shares why MATTERHORN was picked for the plenary and how its findings build on past studies in G/GEJ cancer.
Dr. Pietrantonio discusses how the benefits of T-DXd can potentially reshape the current treatment algorithm.
Dr. Weinberg explains why combining transcriptomic & genomic features can offer an advantage over prior biomarker ...
Dr. Picozzi details PANOVA-3, where TTFields with chemo showed significant survival gains in advanced pancreatic cancer.
Professor Tie discusses DYNAMIC-III, a trial using ctDNA to guide chemo escalation in stage III colon cancer.
Dr. Wainberg details the practice-changing implications of the MATTERHORN study event-free survival data.
Dr. Lieu highlights the randomized trial that looked at atezo with the standard of care for stage III dMMR colon cancer.
Dr. Kelly discusses the first overall survival results from CheckMate 577 and how they compare to previous DFS results.
Dr. Kim discusses survival outcomes of zanidatamab compared to chemotherapy in patients with previously treated HER2+ BTC.
Dr. Finn discusses the follow-up of LEAP-002 and its clinical implications despite not meeting the primary end points.
Dr. Mahalingam shares preliminary results from the randomized phase 2 study of elraglusib with gemcitabine/nab-paclitaxel.













 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.